Akankshi Munjal, PhD (Harvard Medical School)
Assistant Professor of Cell Biology
E-mail: akankshi.munjal@duke.edu
Twitter: @akankshi @munjallab
371B Nanaline Duke Building, Box 3709
Duke University School of Medicine
Durham, NC 27710
Morphogenesis is the process by which simple tissues form complex shapes. Successful embryonic development critically depends on forming proper shapes - failure to do so can be fatal or cause developmental defects. What are the principles of healthy tissue morphogenesis and what are the mechanisms underlying disease etiology? The current framework for tissue morphogenesis explains how genetic and biochemical information drive cellular mechanics and thereby tissue dynamics. This framework, however, fails to account for: 1) Self-organized emerging behaviors that cannot be explained by upstream genetic information; 2) Cell extrinsic information, including extracellular forces; and 3) Multi-scale feedback interactions. We use zebrafish as a vertebrate model system to address these gaps and build a new integrated framework for tissue morphogenesis with generalizable principles.
We deploy integrative approaches, including quantitative imaging, genetics, multi-scale perturbations, theoretical modeling and single cell RNA sequencing, to uncover principles of morphogenesis from zebrafish embryonic tissues. Our current favorite embryonic tissue is the otic epithelium, which, through topological remodeling, gives rise to the adult inner ear (see figure below). Zebrafish, like us, have inner ears comprising of three semicircular canals whose function is to sense balance and whose shape has been conserved through evolution. Inner ear development is a rich platform to study several conventional and unconventional players of tissue morphogenesis including but not limited to cell adhesion, contractility, migration, intercellular & cellular-extracellular matrix (ECM) communication, and pressure & tissue homeostasis. We are currently interested in understanding the roles of the extracellular matrix, multi-scale feedbacks and tissue geometry in driving tissue morphogenesis in a robust, reproducible and self-organized manner.
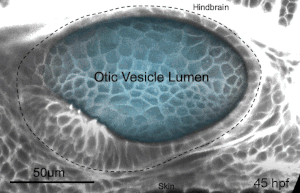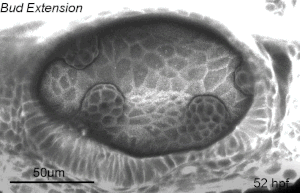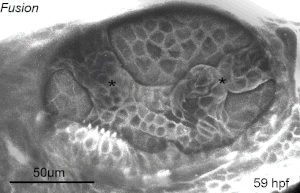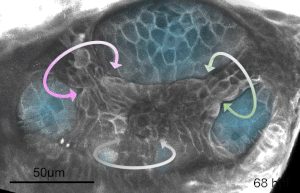

Morphogenesis of the semicircular canals from the otic epithelium
(anterior, posterior and lateral canals labelled as 1, 2 and 3 respectively)
Current Projects
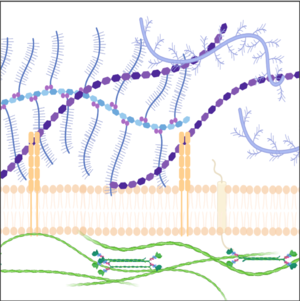
We explore the roles of the ECM in development and disease using imaging (including development of tools for visualizing the ECM), genetics and perturbations approaches.
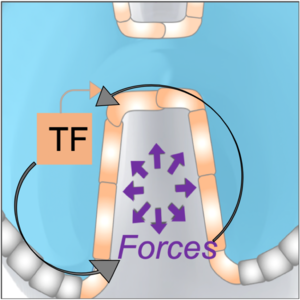
We investigate feedbacks between tissue mechanics and gene-expression using quantitative systems approaches (including biophysical perturbations and physical modeling).
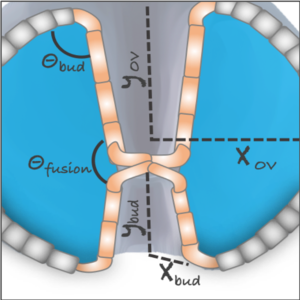
We study the respective contributions of tissue geometry and genetic patterns in robust and reproducible tissue morphogenesis using a combination of experiments and theory.
Positions Available
We are passionate about diversity in forms, science and culture. Our science is collaborative and crosses disciplines. Our culture is integrative and inclusive. Interested candidates should email akankshi.munjal@duke.edu.
Postdocs: Please email a statement of your present and future research interests, CV and contact of two referees.
PhD: Please write to Dr. Munjal to set up a rotation.
Research Technician: We are seeking a technician to contribute to designing and performing experiments, lab supply management and training lab members. Please email a CV, statement of interests, relevant coursework and research experience. Experience with molecular biology and/or zebrafish is preferred.
Recent publications:
- Chugh M*, Munjal A*, Megason SG*. Hydrostatic pressure as a driver of cell and tissue morphogenesis. Semin Cell Dev Biol. 2022 May 6:S1084-9521(22)00145-8. doi: 10.1016/j.semcdb.2022.04.021. (*co-corresponding author)
- Munjal, A.*, Hannezo, E., Mitchison, T. & Megason, S*. Extracellular hyaluronate pressure shaped by cellular tethers drives tissue morphogenesis. Cell, doi: 10.1016/j.cell.2021.11.025 (2021). (*co-corresponding author)
- Banerjee, D. S., Munjal, A., Lecuit, T. & Rao, M. Actomyosin pulsation and flows in an active elastomer with turnover and network remodeling. Nature Communications 8, 1121, doi:10.1038/s41467-017-01130-1 (2017).
- Kerridge, S.*, Munjal, A. *, et al. Modular activation of Rho1 by GPCR signalling imparts polarized myosin II activation during morphogenesis. Nat Cell Biol18, 261-270, doi:10.1038/ncb3302 (2016). (*co-first author)
- Munjal, A., Philippe, J.-M., Munro, E. & Lecuit, T. A self-organized biomechanical network drives shape changes during tissue morphogenesis. Nature 524, 351-355, doi:10.1038/nature14603 (2015).
- Munjal, A. & Lecuit, T. Actomyosin networks and tissue morphogenesis. Development 141, 1789-1793, doi:10.1242/dev.091645 (2014).